The movement of individual organisms is one of the most fundamental features of life and is a crucial component of almost any ecological and evolutionary process that must be considered when dealing with major problems such as habitat fragmentation, climate change, biological invasions, and the spread of pests and diseases. The rich variety of movement modes seen among microorganisms, plants, and animals has fascinated mankind since time immemorial. The prophet Jeremiah (7th century B.C.), for instance, described the temporal consistency in migratory patterns of birds, and Aristotle (4th century B.C.) searched for common features that unified animal movements (Nathan 2008).
Modern movement research is characterized by a broad range of specialized scientific approaches, each developed to explore a different type of movement carried out by a specific group of organisms. Beyond this separation across movement types and taxonomic (or functional) groups, movement research is divided into four different “paradigms” – the random, biomechanical, cognitive, and optimality approaches – each of which is loosely linked to the others. Although movement research is extensive and growing rapidly, specialization comes at a cost: measurement and analysis tools are repeatedly reinvented, and lessons learned from one line of research often do not affect other lines. Most importantly, there is a lack of a cohesive framework that could serve as a unifying theme for developing a general theory of organism movement.
Recently, Nathan et al., (2008) formulated a movement ecology paradigm for unifying organismal movement research. This paradigm places movement itself as the focal theme and aims to promote the development of an integrative theory of organism movement to better understand the causes, mechanisms, patterns, and consequences of all movement phenomena. The framework asserts that four basic components are needed to describe the mechanisms underlying movement of all kinds. The first is the organism’s internal state; this is the multidimensional state (e.g., physiological and neurological) of the focal individual and it affects an individual's motivation and readiness to move. The second component is motion capacity; this is the set of traits (e.g., biomechanical or morphological machineries) that enables the focal individual to execute or facilitate movement. This capacity reflects the organism’s basic ability to move. The third component, navigation capacity, is the set of traits (e.g., cognitive or sensory machineries to obtain and use information) that enables the focal individual to orient its movement in space and/or time. This capacity affects where and when an organism chooses to move. Last but not least is the broad range of external factors that affect movement. These include a wide set of biotic and abiotic environmental factors that affect the movement of the focal individual. The different components of the framework interact to form the animal’s movement path. These components include three processes. The first is the animal’s motion process, the realized motion capacity given the impact that the current location, internal state, and external factors have on the fundamental motion capacity of the focal individual. The second is the animal’s navigation process, which is the realized navigation capacity given the impact of the current location, internal state, and external factors on the fundamental navigation capacity of the focal individual. The third process is the movement propagation process, which is the realized movement produced by the motion process (and optionally affected by the navigation process). Figure 1 offers a graphic representation of these relationships.
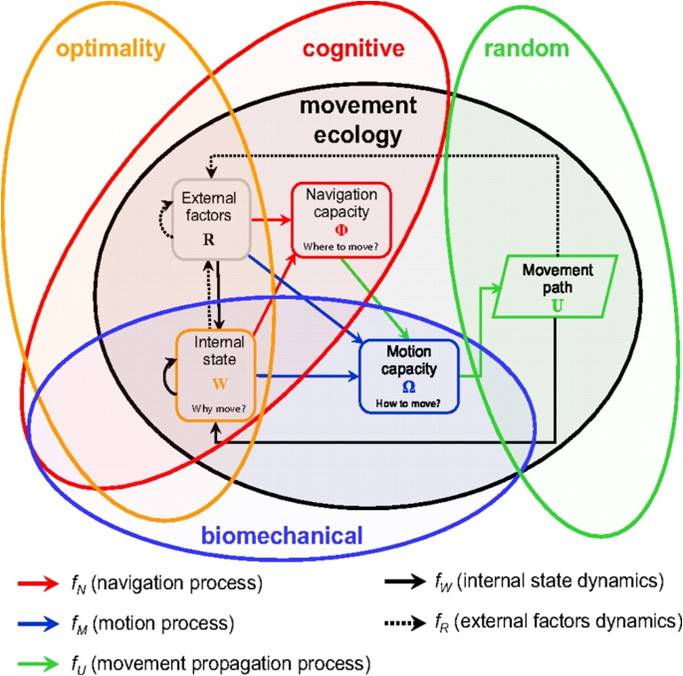
Figure 1. A general conceptual framework for movement ecology, composed of three basic components related to the focal individual (internal state, motion capacity, and navigation capacity) and a fourth basic component referring to external factors affecting its movement. The relationships among components are related to the processes by which they affect each other, with arrows indicating the direction of impact. The resulting movement path feeds back to the internal and external components. The relationships among the proposed movement ecology paradigm and four existing paradigms representing different scientific disciplines in which the movement of organisms is being studied are also shown. Elements in the gray background are components of the movement ecology framework (Taken from Nathan et al. 2008).
At first glance, this framework appears to be primarily applicable to self-propelled sentient animals, for which the internal motivation to move and the ability to move actively while making decisions in response to information about the environment are comprehensible. While this is indeed the most common use of this framework (Holyoak et al. 2008), the framework can also be similarly applied to investigate the movement of passively transported organisms that lack a central nervous system, such as plants (Damschen et al. 2008, Wright et al. 2008). Moreover, in a further development of this framework, Tsoar, Shohami, and Nathan (2011) also demonstrated a twofold nested design of the movement ecology framework (Figure 2). In the inner loop, the dispersed seed is the focal individual and the vector (a fruit bat in their example) is a major external factor affecting the seed’s movement. The seed’s motion capacity is derived from seed retention time in the bat’s digestive tract and movements. The internal driver for the seed movement is the evolutionary advantages of dispersal (such as escaping from the vicinity of the mother plant). In the outer loop, the animal serving as the dispersal vector (the fruit bat) is the focal individual. The bat’s movement pattern is shaped by other factors. For example, hunger may drive the bat to search for food, and the spatial distribution of the fruiting trees may shape the search pattern of the bat, while interacting with its navigation capacity. In other words, modeling seed dispersal requires animals to not only consider the movement ecology of the plant, but also the movement ecology of its vectors. The interplay between the two will ultimately determine the movement path of the plant.
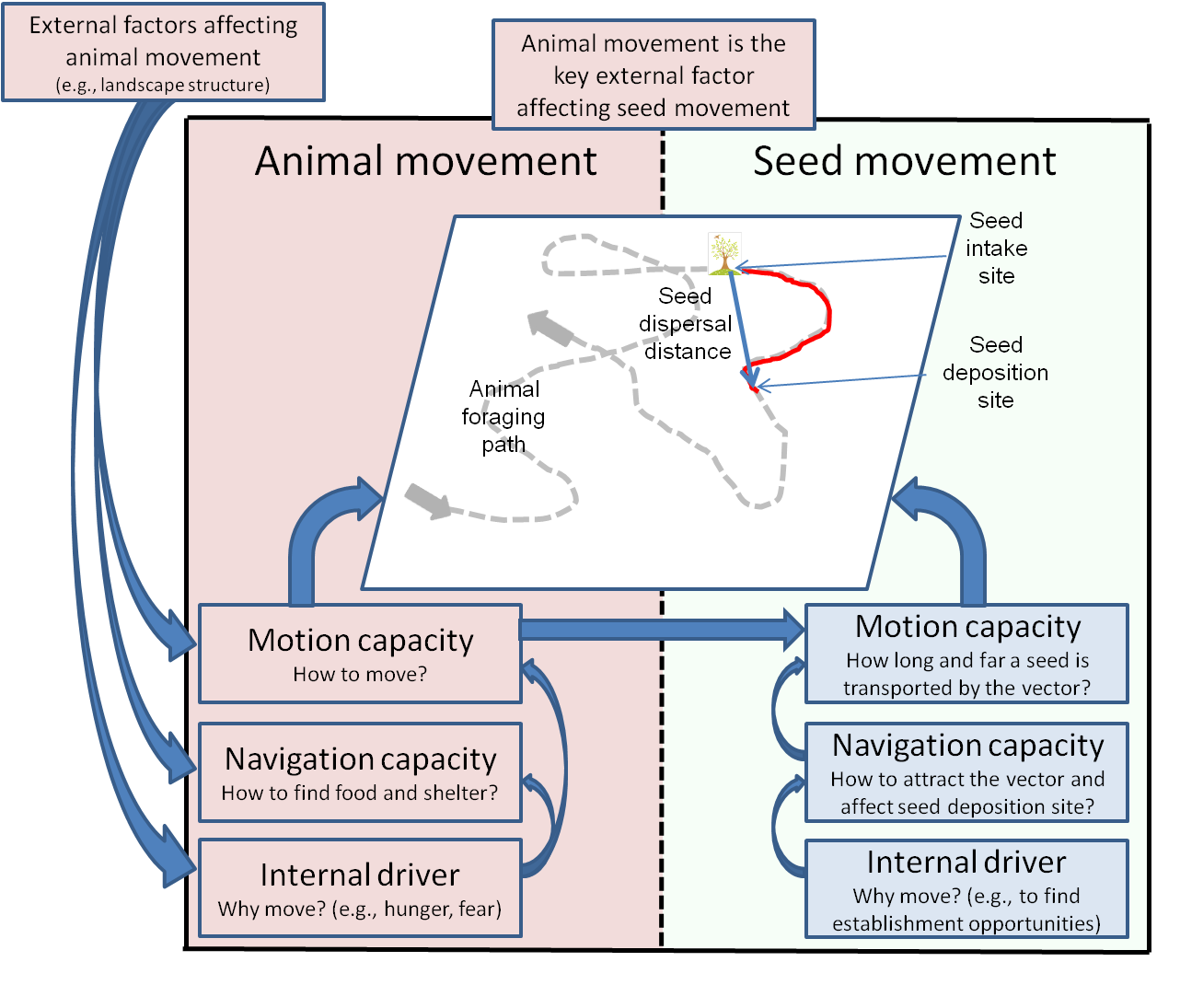
Figure 2. A general conceptual framework for the movement ecology of animal-dispersed plants. The framework has a twofold nested design. In the inner loop, the dispersed seed is the focal individual and the animal (the dispersal vector) is the major external factor affecting its movement. In the outer loop, the dispersal vector is the focal individual. (Taken from Tsoar et al., 2011).
Identifying the relevant crossroads of interactions between the plant and the vector is an area in which the movement ecology framework can greatly help to identify traits and mechanisms that could, at least partially, explain and predict plant dispersal processes. Fruit and seed characteristics interact with the set of frugivores the plant attracts, which may differ in their navigation and motion capacities, resulting in different movement paths of the seeds. For example, synchronization of fruiting with the passage of long-distance migrating animals could favor long distance dispersal (LDD) and population spread, whereas attracting dispersers that consume fruit and rest on the source plant would favor dispersal over shorter distances. This also depends on the seed passage time and, for some animals, on the timing of fruiting (for example, during the breeding season animals may carry seeds back to their offspring or mate) (Tsoar et al. 2011).
Further development of the conceptual framework aims at improving models’ tractability and facilitating the biological interpretation of current movement models (Shimatani et al. 2012). These improvements are achieved by using a recently developed circular, auto-regressive model that also accounts for the asymmetric effects of external factors such as drift caused by winds for handling the distributions of turning angles. Following this approach, Shimatani et al. (2012) were able to separately evaluate the effects of external factors on movement from the animal’s internal state, as demonstrated by their analysis of Streaked Shearwater (Calonectris leucomelas) tracks.
References
-
Damschen, E. I., L. A. Brudvig, N. M. Haddad, D. J. Levey, J. L. Orrock, and J. J. Tewksbury. 2008. The movement ecology and dynamics of plant communities in fragmented landscapes. Proceedings of the National Academy of Sciences of the United States of America 105:19078–83.
-
Holyoak, M., R. Casagrandi, R. Nathan, E. Revilla, and O. Spiegel. 2008. Trends and missing parts in the study of movement ecology. Proceedings of the National Academy of Sciences of the United States of America 105:19060–19065.
-
Nathan, R. 2008. An emerging movement ecology paradigm. Proceedings of the National Academy of Sciences 105:19050–19051. Academic Press Inc, San Diego.
-
Nathan, R., W. M. Getz, E. Revilla, M. Holyoak, R. Kadmon, D. Saltz, and P. E. Smouse. 2008. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America 105:19052–19059.
-
Shimatani, I. K., K. Yoda, N. Katsumata, and K. Sato. 2012. Toward the quantification of a conceptual framework for movement ecology using circular statistical modeling. PLoS ONE 7:e50309: 1-13.
-
Tsoar, A., D. Shohami, and R. Nathan. 2011. A movement ecology approach to study seed dispersal and plant invasion: an overview and application of seed dispersal by fruit bats. Pages 103–120. The nuts and bolts of invasion ecology.
-
Wright, S. J., A. Trakhtenbrot, G. Bohrer, M. Detto, G. G. Katul, N. Horvitz, H. C. Muller-Landau, F. A. Jones, and R. Nathan. 2008. Understanding strategies for seed dispersal by wind under contrasting atmospheric conditions. Proceedings of the National Academy of Sciences of the United States of America 105:19084–9.











